Vitamins and minerals in infant milks
Vitamins and minerals are micronutrients – substances that are essential in the diet in minute quantities for growth, maintenance and functioning. Vitamins and minerals in breastmilk are generally considered to be absorbed more efficiently than those in infant formula and therefore more has typically been added to infant formula than would be found in breastmilk, to allow for reduced absorption levels. The bioavailability of calcium and zinc are known to be significantly less in infant milk compared to breastmilk and some other micronutrients may also have lower bioavailability in infant milks, but data showing lower bioavailablity for some elements such as copper has been disputed (Koletzko et al, 2005). Low iron bioavailability from infant milks has long been assumed. It has been suggested that iron absorption from both breastmilk and infant formula is about 15%-20% (Koletzko et al, 2005), but other expert groups suggest that absorption from human milk is nearer to 50% and from formula 7%-14% (EFSA, 2014).
As some vitamins and minerals can be harmful if supplied in excess, the European Commission Directive on Infant Formulae and Follow-on Formulae currently specifies minimum and maximum levels of vitamins and minerals that must be present in infant and follow-on formula milks. However, the recent EFSA Scientific opinion on the essential composition of infant and follow-on formulae suggests only minimum ‘target’ amounts (EFSA, 2014). Some micronutrients are added to infant milks to achieve the target levels specified by regulations, but some will be present in sufficient quantities within the raw ingredients. As some vitamins deteriorate during storage, infant milk must allow for this in the amounts added at manufacture or include additives that reduce the deterioration. There has been a suggestion by the FAO/WHO Codex Alimentarius Committee that:
“Whenever foods are given to infants under 12 weeks of age, they should be made up from fresh ingredients every day, as infants may not have developed to a point where they are able to cope with substances used to prolong shelf-life that present no problem to adults” (Codex Alimentarius Committee, 2006).
This is obviously not possible, but highlights the fact that additives used for preservation in infant formula are unregulated in relation to their effects on infants. It has also been suggested that babies given the freshest milks might get dangerously high doses of some vitamins, and those getting products stored for long periods might get dangerously low doses (Koletzko and Shamir, 2006). Requests to infant milk manufacturers in the UK to explain how they manage micronutrient contents of milk over time, and how frequently milks are analysed to ensure levels are always safe, have been made, but no manufacturer has as yet provided any information on this.
A summary of information relating to all micronutrients in human milk and the rationale for decisions made on the essential composition of infant and follow-on formula can be found in the EFSA scientific opinion (EFSA, 2014). Below we highlight a number of micronutrients where we feel there are specific interesting points to make.
Calcium
Calcium is an integral part of the skeleton where it has a structural role, and calcium is needed for bone rigidity, strength and elasticity. Calcium deficiency in children leads to inadequate growth and bone deformity. Calcium is present in breastmilk at a level of about 20-30mg/100ml. Calcium absorption efficiency from breastmilk is suggested as about 58%, compared to about 38% from milk-based infant formula in the first four months of life, although other research has suggested breastmilk absorption as high as 76% and absorption from infant formula based on milk protein and hydrolysed protein of around 60% (EFSA, 2014). Absorption will vary depending on formula type and the infant’s age. The minimum (target value) for calcium in infant and follow-on formulae of 50mg/100kcal reflects the potential differences in calcium absorption between breastmilk and infant milk.
It is interesting to note that there are some anomalies between levels set by the European Commission Directive on Infant Formulae and Follow-on Formulae and the UK national dietary recommendations. All infant formulas available in the UK contain levels of calcium that are within the levels set by the Directive. However, the UK dietary reference values set the estimated average requirement (EAR) for calcium for formula fed infants aged 0-12 months at 400mg/day and the reference nutrient intake (RNI) (which meets the needs of 97.5% of the population) at 525mg/day (Department of Health, 1991). Based on a typical first infant milk containing about 50mg calcium/100ml, an infant would be required to consume about 800ml of infant formula a day to achieve the EAR calcium intake, or about 1050ml a day to meet the RNI. Based on Royal College of Nursing feeding guidelines for infants by age, the EAR could be achieved on average at 6-8 weeks and beyond, but the RNI not until 4-5 months and beyond.
Iron
During the first six months of life, infants can obtain sufficient iron from breastmilk or from an appropriate infant formula. There has been considerable discussion about the optimum level of addition of iron to formula and follow-on formula milks, and about the absorption efficiency. The estimate for absorption efficiency of iron from formula currently used by EFSA (2014) is 7%-14%, with an average of around 10%, taken from Quinn (Quinn, 2014). EFSA (2014) also suggest that absorption of iron from human milk could be around 50%, but that this may be lower.
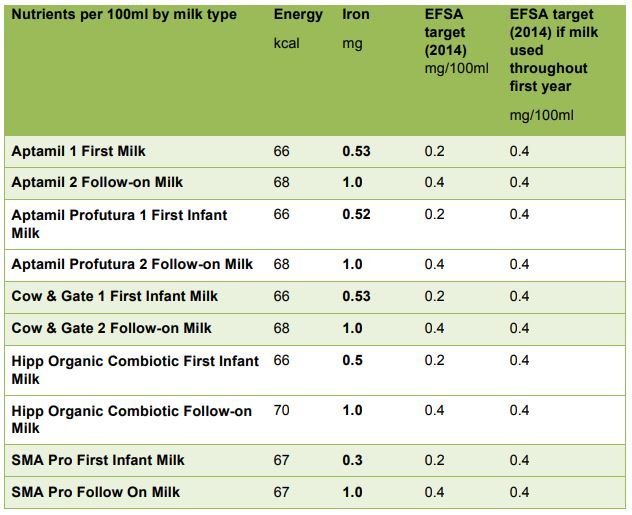
The current minimum and maximum standards for iron in infant formula are 0.3mg1.3mg/100kcal. Average first milks in the UK contain about 0.5mg/100ml, which is almost 7 times higher than the amount found in mature breastmilk. The EFSA Scientific opinion on the essential composition of infant and follow-on formulae (EFSA, 2014) has set minimum (target) values for iron in infant formula at 0.3 mg/100kcal (0.2mg/100ml) and for follow-on formula at 0.6mg/100kcal (0.4mg/100ml). However, they suggest that, if the same formula is to be used from the first months of infancy and be suitable for the whole of the first year, the minimum iron content should be 0.6mg/100kcal (0.4mg/100ml) for milk-based formula (with higher amounts suggested where milks are made from protein hydrolysates). The table below shows that the majority of first infant formula milks available in the UK meet the higher target for iron required for follow-on formula and are therefore, by EFSA criteria, appropriate throughout the first year.
It is however, important to remember that current UK infant feeding guidelines recommend that after 6 months of age additional iron requirements should be met by including iron rich complementary foods in the diet.
Iron content of major-brand first infant formulas suitable from birth and follow-on formulas marketed from 6 months of age, compared to the ‘target’ nutrient values proposed by EFSA (2014)
Follow-on formula can currently have iron contents of between 0.6mg/100kcal and 2.0mg/100kcal, with most brands having a content of about (1mg/100ml). A study in Chile among a sample of infants aged 6-12 months who received little additional iron from complementary foods given fortified formula with either 0.2mg iron/100ml or 1.3mg iron/100ml reported no significant difference in iron deficiency anaemia between the groups (Walter et al, 1998). This suggests that formula with a relatively small amount of iron appear to prevent iron deficiency anaemia. By 6 months of age in the UK, infants will also be receiving other foods, and many of these complementary foods will also be iron-fortified, so there is considerable potential for very high iron intakes. The EFSA (2014) Scientific opinion on the essential composition of infant and follow-on formulae recommends a target (minimum) value of 0.6mg iron/100kcal for follow-on formula, which is equivalent to about 0.4mg iron/100ml (EFSA, 2014).
Follow-on formula have been vigorously marketed as a good source of iron for older infants, but it is agreed that there is no need, and potential risk, from the use of follow-on formula (WHO, 2013) and that it offers no advantage over standard infant formula after the age of 6 months (Moy, 2000). A large study from Chile, which looked at the impact of iron-fortified formula in infants aged 6-12 months on a range of cognitive and learning outcomes at 10 years of age, showed that iron-replete infants given iron-fortified formula did significantly less well in terms of long-term development than similar infants given low-iron formula, or irondeficient infants given high-iron formula (Lözoff et al, 2011). There is also some evidence that high iron intakes among iron-replete toddlers may actually have an adverse effect on growth (Idjradinata et al, 1994) and a large trial of nearly 500 infants and toddlers given follow-on formula between 9 and 18 months of age in the UK found that there were no developmental or growth advantages in children given iron-supplemented follow-on formula (Morley et al, 1999).
Infant formula based on soya protein will contain phytic acid and this may inhibit iron absorption (Hurrell et al, 1992). The minimum and maximum levels of iron in soya protein based formula must therefore be about 1.5 times higher than in those based on cows’ or goats’ milk protein.
Risks associated with high iron intakes in infants
There is now much greater consideration of the potential risks associated with too much iron, which is a potent pro-oxidant and which, in contrast to other nutrients, cannot be actively excreted by humans. The regulation of iron absorption is immature in infants and does not reach adult status until after 9 months of age (Dömellof et al, 2002), which means that, whatever amounts of iron are given, they will be absorbed and accumulated, raising the risk of iron overload. The adverse effects on growth observed when high iron is given to infants may be due to interactions with zinc, and this could also impact on the immune system and be related to infection risk (Koletzko et al, 2005; Iannotti et al, 2006).
There is some evidence of lower copper status and copper absorption in infants fed formula with a higher level of iron (Lönnerdal and Hernell, 1994). There is also some evidence that excessive iron intakes may result in both a reduced uptake of trace metals including copper, and oxidation of lipids, due to the pro-oxidant effects of excess iron (Aggett et al, 2002). There is also some evidence of a negative impact on growth and incidence of diarrhoea for high iron intakes in children aged 4-9 months who have adequate iron status (Dewey et al, 2002), and a number of other studies have reported a negative impact on growth for high iron intakes in iron-replete children (Idjradinata et al, 1994; Majumdar et al, 2003; Lind et al, 2008).
EFSA (2014) suggests the following:
“Studies support that the absorption of iron cannot be down-regulated before the age of nine months with a risk for overload in those infants with sufficient iron stores but high iron intakes. Iron replete infants might therefore be at risk of negative health consequences if given extra iron.”
Vitamin D
Vitamin D is essential for bone health in infancy. Current UK Government advice is that pregnant women in the UK take vitamin D supplements in pregnancy and when breastfeeding and that breastfed babies receive 8.5-10µg of vitamin D as a supplement from birth as a prudent measure. Vitamin D is added to infant milks in the form of vitamin D3 (cholecalciferol), and infant formula provides enough vitamin D for the first year of life if infants are receiving 500ml or more per day. The minimum (target) vitamin D content for infant and follow-on formula is 2µg/100kcal (EU 2016/127).
Manufacturers of fortified milks for children over 1 year of age suggest in marketing material that these milks can protect children at risk of low vitamin D intakes, and correctly point out that vitamin D is likely to be available in limited quantities in many toddler diets. It is, however, recommended that all children aged 1-4 years in the UK have daily vitamin drops containing vitamins A, C and D and these are available through the Healthy Start scheme in many areas (www.healthystart.nhs.uk). In addition, it is recommended that babies and children spend some time safely outside in the summer sun (following guidance on sun safety) as they will make some vitamin D through the action of summer sunlight on the skin. The use of fortified milks for children over 1 year of age is not recommended in the UK, as these milks are high in sugar and can be low in some other nutrients that milk importantly provides.
Koletzko and colleagues at the Early Years Nutrition Academy suggest that in many areas there are concerns over the vitamin D status of older children and that a higher maximum vitamin D content for follow-on formula may be useful (Koletzko et al, 2012). However, they make the point that, where there are vitamin D supplementation programmes in place, the vitamin D content of follow-on formula should be below maximum values. The current regulations make no differentiation between infant and follow-on formula in terms of vitamin D content and have increased the minimum requirement of vitamin D in both infant and follow-on formula from 1µg/100kcal (0.67µg/100ml) to 2µg/100kcal (1.35µg/100ml) (EU 2016/127).
References
Aggett P, Agostoni C, Axelsson I, et al (2002). Iron metabolism and requirements in early childhood: do we know enough? A commentary by the ESPGHAN Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition, 34, 337-345.
Codex Alimentarius Committee (2006). Joint FAO/WHO Food Standards Programme. Codex Committee on Nutrition and Food for Special Dietary Uses. 28th Session. Proposals for Sectors of Working Group on Food Additives. CX/NFSDU/06/28/4-Ad.
Department of Health (1991). Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. Report on Health and Social Subjects 41. London: The Stationery Office.
Dömellof M, Lönnerdal H, Abrams AS, Hernell O (2002). Iron absorption in breast-fed infants: effects of age, iron status, iron supplements and complementary foods. American Journal of Clinical Nutrition, 76, 198-204.
European Food Safety Authority (2014). Scientific opinion on the essential composition of infant and follow-on formulae. EFSA Journal, 12 (7), 3760. Available at http://www.efsa.europa.eu/en/efsajournal/doc/3760.pdf
Hurrell R, Juillerat M, Reddy M, et al (1992). Soya protein, phytate and iron absorption in humans. American Journal of Clinical Nutrition, 56, 573-578.
Iannotti LL, Tielsch JM, Black MM, et al (2006). Iron supplementation in early childhood: health benefits and risks. American Journal of Clinical Nutrition, 84, 1261-1276.
Idjradinata P, Watkins WE, Pollitt E (1994). Adverse effect of iron supplementation on weight gain of iron-replete young children. The Lancet, 343, 1252-1254.
Koletzko B, Baker S, Cleghorn G, et al (2005). Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated International Expert Group. Journal of Pediatric Gastroenterology, 41, 584-599.